|
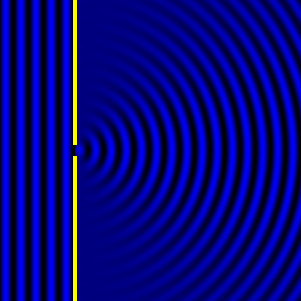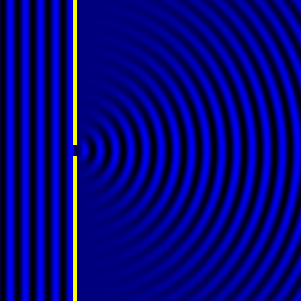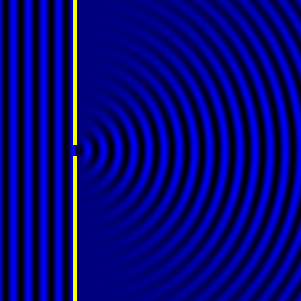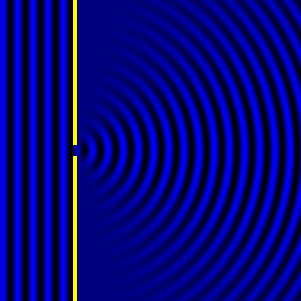
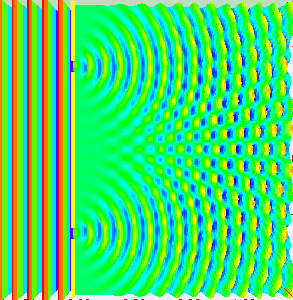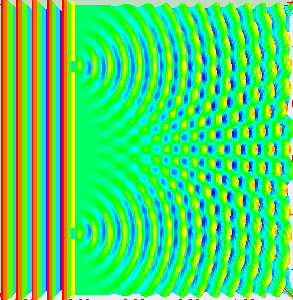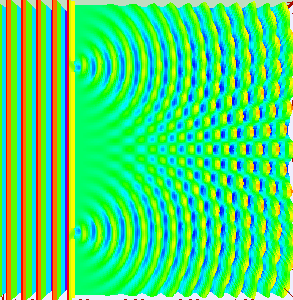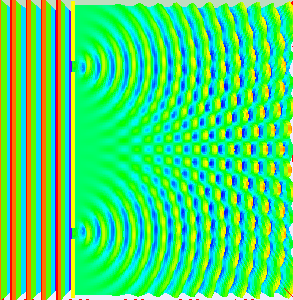
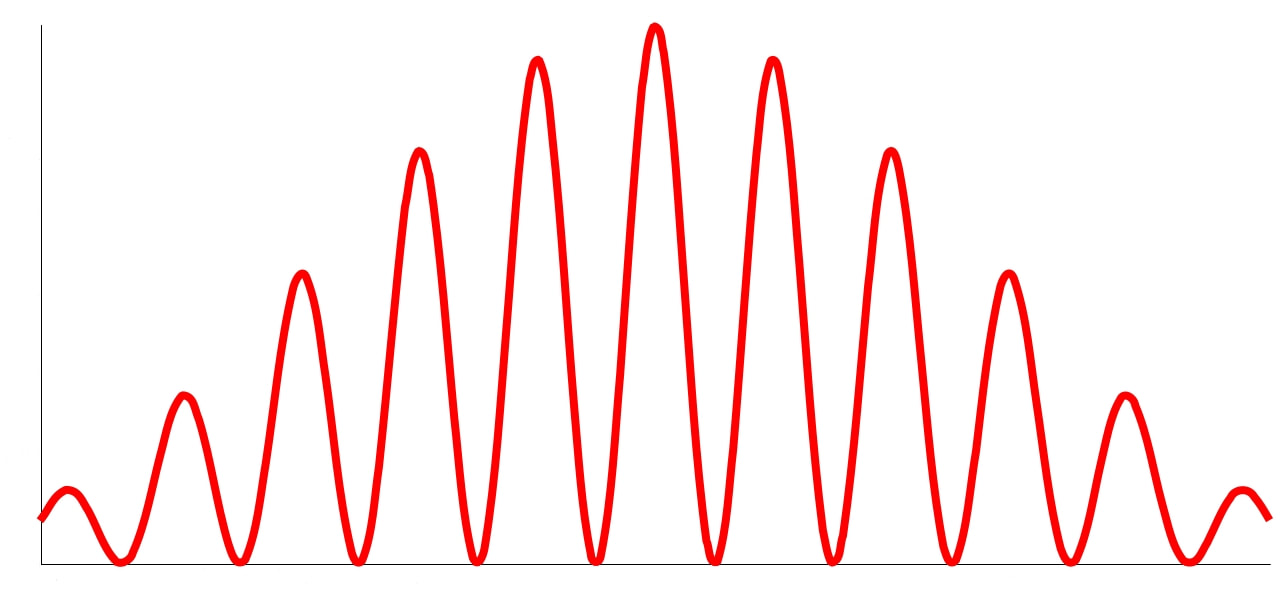
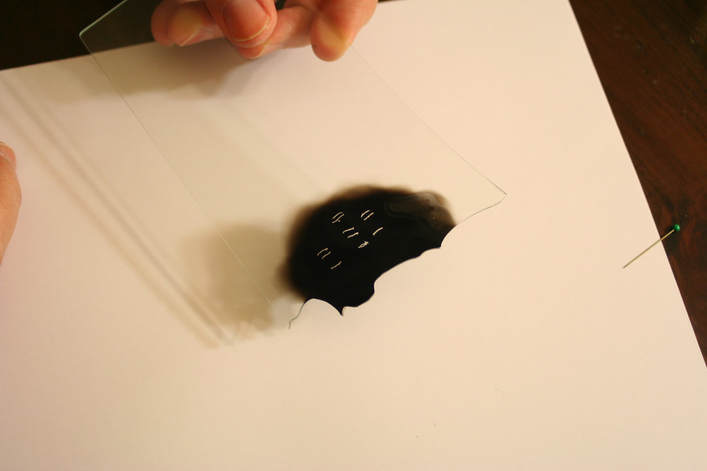
In the late 1700s there was a lot of discussion in scientific circles regarding whether light was a particle or a wave. It was then that a British scientist named Thomas Young designed a famous experiment to settle the matter. Young reasoned that if light were a wave, then two streams of light that interact with each other would generate an interference pattern. What is this? First let’s look at the behavior of water waves. In the simulation below (Figure 1) there are waves that originate on the left side of the image (each of the blue lines represents the crest of a wave, whereas the dark space in between is the trough). These waves hit the yellow wall which has one opening or slit. The part of the wave that makes contact with the slit passes through and moves on to the right side spreading out in a radial pattern. However, when there are two slits, the waves originating from the two slits overlap with each other. In those areas where the crest of one wave intersects the crest of the other, the wave is taller. In those areas where the crest of one wave intersects the trough of the other, they cancel each other out. And in those areas where the trough of two waves meet, the wave is deeper. The pattern they form is called an interference pattern (Figure 2). If you were able to continuously record the waves arriving at the right hand side, you would see a large central swell where the waves originating from both slits reinforce each other, flanked by troughs where they cancel each other out, followed by progressively smaller swells where the intensity of the reinforcement of the waves diminish as they spread out. It would look something like Figure 3. Young expected that if light behaves like a water wave, and were forced through two slits, it would generate a similar interference pattern. On the other hand, if light is made up of particles, the light particles would go through the slits in a straight line generating two distinct images on the right hand side (no interference pattern). I decided to try to perform this experiment to see what results I got, and you can too! When Young performed the experiment 217 years ago he used sunlight, but we will use a laser pointer. Additionally (see below), among other things, we will need a flat glass, a candle, a pin, and matches (not meant as a product endorsement, just matches we’ve had for almost 20 years and barely used). Light the candle and place it on a surface such as a plate and cover one of the sides of the glass with soot. Don’t put the glass in contact with the candle flame for an extended period of time like I did (my glass shattered)! Allow for some cooling in between intervals of exposure to the candle flame. I finally covered one side of my broken glass in soot (careful with those sharp edges if there are any). Shine the laser pointer into the soot-stained surface to make sure light doesn’t go through. Proceed to make single and double slits on the glass’s soot-stained surface with the pin. You can aid yourself with a magnifying glass. This is how my slits looked. In theory they should be straight and as close as possible to each other, but this is the best I could do. I took advantage of a wooden ornament to set up the glass. I ended up holding the laser pointer with my hand, but it’s better if you can hold it in place say with a clamp. Be mindful not to look at the laser pointer straight on, it can damage your eyes! I darkened the room and shone the laser pointer through the single and double slits in the glass into a wall some 15 feet away, and I had another person take pictures with a smart phone. So what were the results? When I shone the laser pointer through a single slit, I obtained the pattern below. So if I were to shine the laser pointer through the double slits, I would expect two such blobs of light next to each other, if light behaves like a particle, right? But when I shone the laser pointer through the double slits, I obtained this: Those blobs of light flanking the central one and decreasing in intensity towards the sides are characteristic of the interference pattern (see Figure 3) that you would expect if light behaves like a wave! Of course, my experimental setup and slit construction were rather crude. Physicists working with more sophisticated equipment can obtain much better patterns. So did Young’s experiment settle the argument? Unfortunately at the time some heavyweights in science such as Isaac Newton favored the particle theory, so Young’s double slit experimental result was slow to catch on. However, in the end things turned out to be more complicated than they seemed (they always do). Not only did light display the behavior of a particle in other experiments, but other entities besides light such as electrons and even some large molecules that no one would ever think of as a wave were found to exhibit both particle and wave behavior. Nowadays light is thought of as having a particle-wave duality (it can exhibit properties of both waves and particles but not at the same time). However, the ingenious experiment designed by Young was improved upon in modern times and ended up generating some of the remarkable results that form the cornerstone of the revolutionary theory that nowadays we call quantum mechanics. And I just performed this experiment in my living room, and you can too! Now, isn’t that cool? Single and double slit GIFs by Lookang used here under an Attribution-Share Alike 3.0 Unported license. Wave by Klaus-Dieter Keller modified and used here under an Attribution 3.0 Unported license.
0 Comments
The popular view of scientists is that of methodical and logical individuals. The scientist evaluates the available data regarding some problem or question of importance, comes up with hypotheses, designs and carries out experiments or observations, processes the results, and comes up with answers. Indeed the scientific pursuit is often comprised of systematic, coherent, and incremental approaches to uncovering the ways of nature, but the view that this is the only way scientists operate is a simplification of the complexity of scientific thinking. Science is replete with stories of madness and passion, stubbornness and irrationality, perilous quests and hopeless struggles, and ego trips and selfless deeds. And this should not surprise anyone. Scientists are, after all, human, and as humans they are affected by the same emotions and contradictions that afflict all human beings, and they often get their ideas by the same means that most non-scientists do. One of these methods of obtaining ideas is no other than dreams. Many scientists of renown have gained inspiration for their work from dreams. The German chemist August Kekule had been trying to figure out the structure of an organic compound called benzene. One day when he dozed off, he dreamt of carbon atoms that joined each other forming a chain. Then the chain of atoms turned into a snake, and the snake bit its tail and started spinning. Kekule woke up and realized that the structure of benzene must be cyclical as opposed to linear. This insight turned out to be true and paved the way for the creation of a new branch of chemistry (aromatic chemistry) and a better understanding of the nature of chemical bonds. The German pharmacologist Otto Loewi had been trying to figure out how nerve signals are transmitted. One night he had a dream of how he could figure this out, and he promptly went to the lab to perform the experiment. He dissected a frog’s beating heart with the vagus nerve still attached and placed the heart in a saline solution. Loewi then proceeded to stimulate the nerve by electrical means, and this had the effect of slowing down the beating heart. He took the solution that bathed the first heart and added it to another’s frog beating heart that he had dissected with no vagus nerve attached to it, and the second heart slowed down too! Loewi had discovered a nerve signal that was released from the stimulated nerve in the first heart and which leaked out to the bathing solution. This chemical signal was still strong enough to be able to act on the second heart when the bathing solution was placed in contact with it. The chemical he discovered that day was eventually shown to be what we today call acetylcholine. Loewi received the Nobel Prize for his discovery in 1936. The Russian Chemist Dmitri Mendeleev was trying to come up with a way to classify all the known elements according to their chemical properties. He had a dream of a table where the elements were arranged in a certain order. When he woke up he quickly wrote it down. Not only was he able to organize all the known elements, but he was able to predict that new elements would be discovered, and he listed their properties. He was eventually proven right and his discovery, the periodic table, is the pillar of modern chemistry. The brilliant Indian mathematician Srinivasa Ramanujan made important contribution to several fields in his discipline. He was a very religious person and claimed that many of his mathematical discoveries were presented to him in dreams by the Hindu god Narasimha. Another mathematician, the American Donald Newman, who made important advances in mathematical areas such as approximation theory, had some approaches for solving problems revealed to him in dreams where he interacted with both known and unknown people. The British naturalist Alfred Russel Wallace dreamt about the theory of evolution by natural selection while hallucinating in the middle of a bout of malaria. He is considered a codiscoverer of the theory along with Charles Darwin. The above, of course, are just some of the big names. Many average scientists have also benefited from their treks to the land of dreams. So how does this square with the idea that science is methodical, logical, and rigorous? The answer is that the scientific method provides a framework for testing ideas. This is the methodical, logical, and rigorous part of science. However, there is nothing in the scientific method regarding what the source of these ideas should be. Dreams, intuition, messages from God or from “beyond”, you name it. When it comes to ideas, anything is fair game for scientists. However, it is what comes after the conception of an idea that makes science what it is. Every idea, no matter its origin, must be put to test. And the horrible little secret of science is that most ideas crash and burn when measured up to the evidence. It is great to read about a scientist like Loewi coming up with a Nobel Prize winning idea in a dream, but the truth is that stories like those of Loewi, Kekule, Mendeleev, and others are the exception rather than the rule. The vast majority of scientists experience repeatedly during their careers what the British biologist Thomas Huxley called “the tragedy of science”: the slaying of a beautiful hypothesis by an ugly little fact. But despite the slain hypotheses, scientists dream on! Scientific discoveries, even important ones, are normally published in specialized journals, and then it’s mostly up to the public relations people, journalists, bloggers, and other pundits to step in, simplify the story, and distill the meaning and importance of the findings through printed, electronic, or televised media letting the public know how “exciting” these findings are. When these discoveries are presented to regular folk, there are no strobe lights, stage fog, explosions, or musical backgrounds. Most of the time there is no show, no drama, no razzle-dazzle. In an age when the attention of the public is only captured by some of the most eye catching, awe inspiring, or just plain outrageous antics and theatrical gimmicks, for the average person science comes across as a pretty boring enterprise. That is not to say that established science can’t be presented in an interesting and exciting way that captures our attention. What I am talking about is science as it happens. Those WOW! moments, those instants in science where something new is discovered or thought about on the spot. Some past WOW! moments that come to mind are the discovery of the correct pairing between the bases of DNA by James Watson, the discovery by Alexander Fleming of the substance secreted by a mold that killed bacteria in a culture (penicillin), the discovery of X-rays by Wilhelm Conrad Rontgen, the discovery of right and left handed crystals of tartrate by Louis Pasteur that gave rise to the discipline of stereochemistry, and the discovery of the periodicity of the elements by Dmitri Mendeleev (periodic table). However, WOW! moments in science are often private affairs (things that happen in the solitude of a lab or an office), and the raw science involved would not be readily understood by any non-scientist bystanders at the time. Unfortunately, the closest we can get to viewing such moments are documentaries or movies where the original scientists, or actors playing those scientists, recount or reproduce the science for a film crew. This often takes place years after the fact. But there is one particular WOW! moment that comes to my mind that was televised live, although to be fair it was a reenactment of something the scientist had discovered a few hours before on his own.  In 1986 the space shuttle Challenger blew up after takeoff, killing 5 NASA astronauts and 2 payload specialists, one of whom was to be the first “teacher in space”, Christa McAuliffe. A presidential commission (the Rogers Commission) was formed to investigate what led to the Challenger disaster, and this commission included the Nobel Prize winning physicist Richard Feynman. Feynman, who had one of the keenest minds of the 20th century and who had tackled some of the great problems in physics, also was a down to earth regular kind of guy who could talk to regular people and understand their problems. His “no bullshit” attitude soon made him a pain for the committee directors. While the members of the committee were in Washington listening to presentations by NASA executives, Feynman was down in Cape Canaveral mixing with the regular engineers and technicians. He soon found out that there was a disconnect between management and their underlings. As it turns out, many of the shuttle launches which were presented by NASA executives as “safe” had really been precarious, with several things going wrong that could have doomed the missions. Up to the launch of Challenger, NASA had just been lucky. One particular area of concern were some rubber seals called O-rings which were intended to plug the joints between the segments of the shuttle’s solid rocket boosters. If these seals failed, the gases inside the booster could leak out disrupting the structure. For their correct functioning, the O-rings had to be resilient. Feynman’s approachability and capacity and desire to understand the truth allowed individuals within the hierarchy of NASA and their contractors to anonymously relay information to him that there had been issues with the O-rings in several flights, and that the possible lack of resiliency of these seals at low temperatures was a particular concern. This was significant as the day Challenger was launched had been one of the coldest among all the shuttle launches. Feynman proceeded in true scientific fashion to perform an experiment. He obtained a clamp at a hardware store, twisted a piece of an O-ring sample, and dunked it in an ice water glass to simulate the temperature of the morning of the Challenger launch. After a few minutes when he removed it and released the hold of the clamp, the O-ring failed to return to its previous shape for several seconds. This indicated that at cold temperatures the O-ring became stiff: it lost its resiliency.
Several hours later at a conference with all the cameras aimed at him (you can see Feynman talking at 1 min and 57 seconds in the video) he reproduced his experiment demonstrating that the O-ring loses its resiliency at cold temperatures and ended his demonstration with the understatement “I believe that has some significance for our problem.” Here is the WOW! moment that reveals the essence of science for all to see. And no lab filled with an army of technicians and postdoctoral and graduate students, or multimillion dollar grants, or fancy and expensive high tech equipment was required: just a man, a clamp, and a glass of ice water. That is all it took to solve the problem of why the Challenger shuttle exploded and expose the folly of an organization that had pushed forward ignoring critical safety problems that led to the death of 7 humans beings. All of this is even more remarkable if you consider that Dr. Feynman was battling cancer at the time he performed his work for the Rogers Commission. He died on February 15, 1988. His account of the investigation of the Challenger disaster and other stories are presented in his book "What Do You Care What Other People Think?": Further Adventures of a Curious Character. The image of the Challenger shuttle taking off by NASA is in the public domain. |
Details
Categories
All
Archives
June 2024
|







 RSS Feed
RSS Feed