|
Many industries and laboratories work with compressed gas cylinders. A typical compressed gas cylinder filled to a pressure of 2,400 pounds per square inch (PSI) will contain a volume of gas, that at atmospheric pressure would occupy nearly 300 cubic feet, compressed into a volume of almost 2 cubic feet. This represents a huge amount of potential energy which, if released suddenly, can have catastrophic consequences. For the dispensation of gas, these cylinders have a valve in one of their ends to which a regulator is attached. This valve is the weakest area of the cylinder. Its rupture can essentially turn the cylinder into a missile that can cause serious property or bodily harm, and the feats of errant gas cylinders have a storied lore in science and industry that includes claims of such cylinders going through walls. Such a claim was examined by the folks at Myth Busters in the video below. A real case of a compressed gas cylinder in an industrial setting which was handled in an unsafe manner can be seen in the video below. The cylinder toppled over breaking its valve, and the resulting explosive release of gas made it go airborne! An additional hazard occurs if the release of gas takes place in an enclosed not very well ventilated space. A gas in the cylinder such as nitrogen can displace all the oxygen-containing air and produce asphyxia. To avoid these situations compressed gas cylinder handlers have follow specific safety protocols.
0 Comments
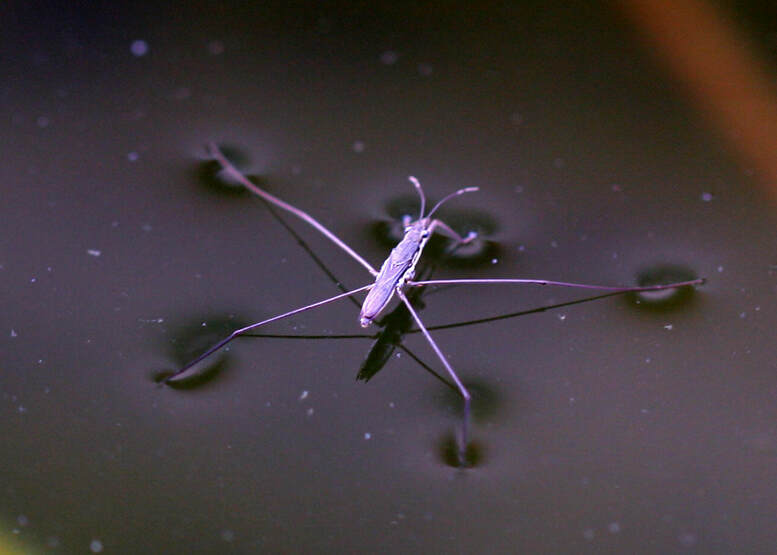
Some plants have evolved the capacity to use insects and other organisms for food, which allows them to live in nutrient-poor environments. To this end they have leaves that have been modified into specialized appendages that trap and digest their prey. One such plant is the pitcher plant, shown below, from the genus Nepenthaceae. This plant has a cup like structure (the pitcher) that has rims and inside surfaces with the consistency of a slippery wax. Insects that venture too far into the pitcher fall into a pool of liquid with digestive enzymes at the bottom of the pitcher and can’t get out. Some pitcher plants have pitchers large enough to trap small animals like mice! Whereas the pitcher plant is a passive trap, other carnivorous plants like the sundews, which belong to the genus Drosera, have a more active involvement in capturing their food. These plants have leaves sporting tiny spine-like protuberances tipped with a droplet of a sticky liquid. Any insect landing on the leaf gets stuck and triggers a response whereby the leave curls about itself, further trapping the insect, and also secreting digestive enzymes. But the most active carnivorous plant is certainly the Venus flytrap (Dionaea muscipula). The Venus flytrap has hinged appendages with open surfaces crowned with hairs at the edges. The inside of the surfaces have tiny hairs which serve as triggers. When an insect brushes against the tiny hairs, the 2 surfaces close upon the insect and the hairs at the edges interlock to prevent their scape. As the insect is pressed between the surfaces, the plant secretes enzymes that digest the hapless critter. If you want to see some of the carnivorous plants mentioned above in action, watch the video below from Gross Science. The pictures of the pitcher plants in a Walmart store in Florida, and the sundew and Venus flytrap plants at the Rawlings Conservatory in Baltimore are by the author and can be used with permission. Featured in this video is a specimen of the peacock mantis shrimp (Odontodactylus scyllarus) filmed at the National Aquarium in Baltimore. Mantis shrimps, which are neither mantises nor shrimps, are a type of crustacean called stomatopods. They are mostly famous for their hard hitting club-like appendages which can shatter the carapace of crabs or crack seashells, and even shatter the glass of aquarium tanks. But mantis shrimps are also remarkable for their capacity to detect ultraviolet light, which humans being lack. In fact, these crustaceans have a total of six distinct structures in their eyes which allow them to discriminate between several wavelengths in the ultraviolet range. To imagine what this would look like check my post on color coded thermal imaging where different wavelengths of light in the infrared range are assigned a color to allow the discrimination between different levels of heat radiance. What features of the marine environment or its inhabitants do these organisms perceive that require such discrimination remains a mystery. Water molecules are made up of one atom of oxygen bonded to two atoms of hydrogen (H2O). When atoms become bonded, they share electrons. However, the oxygen atom is so large compared to the hydrogen atoms that is tends to “hoard” these electrons to a greater extent. This hoarding of the electrons confers upon the oxygen atom a partial negative charge, and conversely, the electron deficit confers upon the hydrogen atoms a partial positive change. Thus water molecules tend to stick because the positive and negative charges attract each other. This creates the phenomenon known as surface tension. Surface tension is an important property of water that living things such as the insect known as water strider exploit. Surface tension and the effects that soap has on it can be used to perform some fun experiments as Physics Girl shows in the video below.
Water strider image by Alexander used here under an Attribution-ShareAlike 2.0 Generic (CC BY-SA 2.0). |
Details
Categories
All
Archives
June 2024
|



 RSS Feed
RSS Feed